Upcoming Events

Statistics for Quality Control & Quality Assurance Practice 3rd Edition
April 23rd- 25th 2024

GCP Quality Management Systems (QMS) from the FDA Perspective 2nd Edition
May 8th – 9th 2024

Annex 1 GMP Compliance - Exploring 15 Key Topics with High Impact Post-2023
May 22nd – 24th 2024

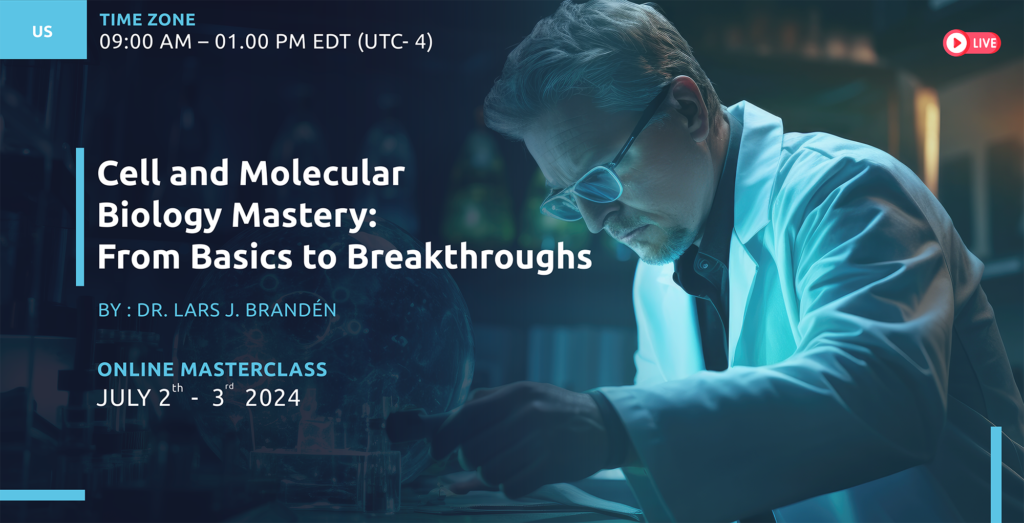
CMC For Cell and Gene Therapy with a focus on viral vectors 2nd edition
June 4th – 6th 2024

Successful Site Transfers for Manufacture & Quality Control (EU Requirements)
June 25th – 26th 2024

Mastering Pharmaceutical Standards with Advanced GCP QMS Techniques
June 25th – 26th 2024

Mastering Particle Size Characterization - Integrating Laboratory Excellence
July 17th – 19th 2024
Upcoming Events
Watch the seconds elapse in
anticipation of the approaching event
Days
Hours
Minutes
Seconds
Imminent Events

Statistics for Quality Control & Quality Assurance Practice 3rd Edition
April 23rd- 25th 2024

GCP Quality Management Systems (QMS) from the FDA Perspective 2nd Edition
May 8th – 9th 2024